Navigating Trends in Oral Solid Dosage Contract Manufacturing
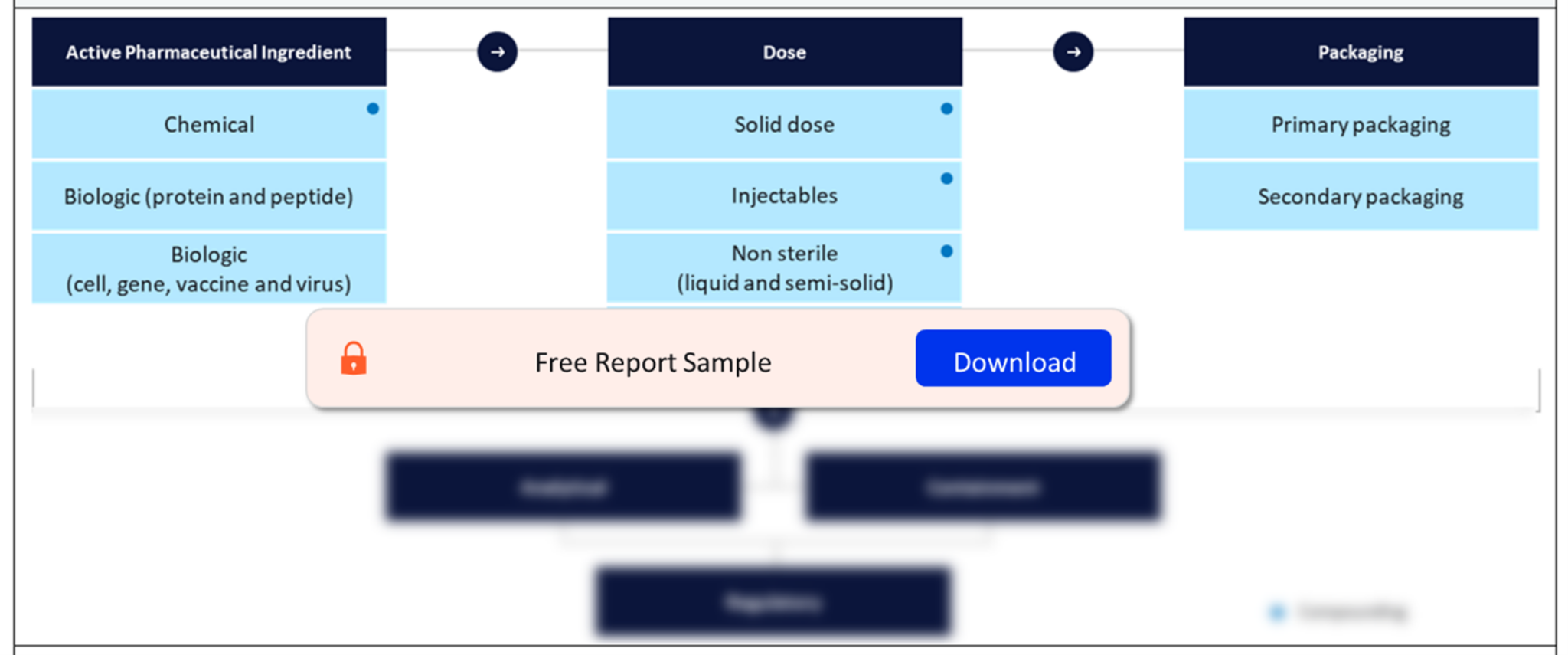
Our Oral Solid Dosage Contract Manufacturing analysis explores cutting-edge trends, innovations, and the evolving landscape of outsourcing in the pharmaceutical industry. Stay informed and trust as your guide in understanding the dynamic realm of oral solid dosage contract manufacturing. The Contract Pharmaceutical Dose Manufacturing Industry report is a comprehensive look at the finished dose contract manufacturing landscape in 2023.
Buy the Full Report for More Insights on the CMO Industry Trends, Download a Free Report Sample
Embark on a pharmaceutical journey through precision and efficiency—the Oral Solid Dosage (OSD) Contract Manufacturing Market. In this detailed analysis, we navigate the landscape of pharmaceutical manufacturing, exploring the trends, innovations, and strategic outsourcing dynamics that define the realm of OSD contract manufacturing.
1. Outsourcing Trends in Pharmaceuticals:
Delve into the evolving trends of outsourcing within the pharmaceutical industry. Explore the shifting dynamics that drive companies to leverage external expertise for the manufacturing of oral solid dosage forms, from tablets to capsules and beyond.
2. Market Overview and Key Players:
Navigate the landscape of OSD contract manufacturing. Identify key players, market dynamics, and the competitive environment that shapes the outsourcing market for oral solid dosage forms. Gain insights into the strategic alliances and expansions driving industry growth.
3. Technological Advancements in OSD Manufacturing:
Explore the cutting-edge technologies revolutionizing OSD manufacturing. From advanced equipment for tablet compression to innovations in coating and packaging, understand how technological advancements enhance efficiency, quality, and compliance in pharmaceutical production.
4. Regulatory Compliance and Quality Assurance:
Uncover the critical aspects of regulatory compliance and quality assurance in OSD contract manufacturing. Explore the stringent standards, cGMP (current Good Manufacturing Practice) requirements, and the role of regulatory bodies in ensuring the safety and efficacy of pharmaceutical products.
5. Formulation Development and Customization:
Examine the formulation development processes and customization options available in OSD contract manufacturing. Understand how manufacturers tailor formulations to meet specific drug requirements, addressing factors such as bioavailability, stability, and patient adherence.
- Questions and Answers
- Opinion
- Motivational and Inspiring Story
- Technology
- Live and Let live
- Focus
- Geopolitics
- Military-Arms/Equipment
- Securitate
- Economy/Economic
- Beasts of Nations
- Machine Tools-The “Mother Industry”
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film/Movie
- Fitness
- Food
- Jocuri
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Alte
- Party
- Religion
- Shopping
- Sports
- Theater
- Health and Wellness
- News
- Culture

