Orphan Drugs Market Size Share, Growth & Demands
IMARC Group, a leading market research company, has recently released a report titled “Orphan Drugs Market Report by Drug Type (Biological, Non-Biological), Disease Type (Oncology, Hematology, Neurology, Cardiovascular, and Others), Phase (Phase I, Phase II, Phase III, Phase IV), Top Selling Drugs (Revlimid, Rituxan, Copaxone, Opdivo, Keytruda, Imbruvica, Avonex, Sensipar, Soliris, and Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Stores, and Others), and Region 2025-2033”. The study provides a detailed analysis of the industry, including the global orphan drugs market share, trends, size, and industry growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
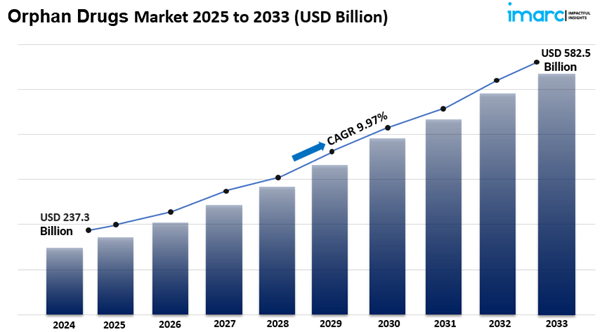
The global orphan drugs market size reached USD 237.3 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 582.5 Billion by 2033, exhibiting a growth rate (CAGR) of 9.97% during 2025-2033.
Request to Get the Sample Report:
https://www.imarcgroup.com/orphan-drugs-market/requestsample
Orphan Drugs Market Trends in 2025
A prominent trend in the orphan drugs market is the rise of personalized medicine tailored to the unique genetic profiles of patients with rare diseases. As advancements in genomics and biotechnology progress, there is an increasing emphasis on developing therapies that are specifically designed for individual patients. By 2025, it is anticipated that personalized orphan drugs will gain traction, allowing for more effective and targeted treatments. This trend is driven by the growing understanding of the genetic underpinnings of many rare diseases, enabling researchers to develop therapies that address the specific mechanisms of action involved.
Additionally, the integration of data analytics and artificial intelligence in drug discovery is facilitating the identification of potential drug candidates that align with patients' genetic profiles. As a result, pharmaceutical companies are investing in precision medicine initiatives, leading to the development of bespoke therapies that not only improve patient outcomes but also enhance the efficiency of clinical trials. This shift towards personalized medicine is expected to reshape the orphan drugs landscape, fostering innovation and ensuring that patients receive treatments that are both effective and tailored to their individual needs, thereby driving growth in the orphan drugs market.
Market Dynamics of the Orphan Drugs Market
Increasing Prevalence of Rare Diseases
A significant dynamic in the orphan drugs market is the increasing prevalence of rare diseases, which is driving demand for targeted therapies. As advancements in genomics and biotechnology continue to unveil the genetic basis of various rare conditions, more diseases are being identified and classified as rare. By 2025, it is expected that the number of recognized rare diseases will grow, leading to a corresponding increase in the patient population requiring specialized treatments. This trend is further fueled by improved diagnostic capabilities, allowing for earlier and more accurate identification of rare diseases.
As awareness of these conditions rises among healthcare professionals and patients, the demand for orphan drugs—medications specifically developed to treat these rare diseases—is expected to escalate. Pharmaceutical companies are increasingly investing in research and development (R&D) for orphan drugs to address unmet medical needs, resulting in a growing pipeline of new therapies. This heightened focus on rare diseases not only enhances treatment options but also encourages collaboration between stakeholders, including biotech firms, academic institutions, and patient advocacy groups, to accelerate drug development and improve patient access to necessary therapies.
Regulatory Incentives and Support for Orphan Drug Development
Another crucial dynamic influencing the orphan drugs market is the regulatory incentives and support provided by government bodies to stimulate the development of orphan drugs. Many countries have implemented legislation that offers various incentives, such as tax credits, grants, and extended market exclusivity, to encourage pharmaceutical companies to invest in R&D for rare diseases. By 2025, it is anticipated that these regulatory frameworks will continue to evolve, providing even greater support for orphan drug development. The Orphan Drug Act in the United States and similar regulations in Europe and other regions have successfully fostered an environment conducive to innovation, resulting in a significant increase in the number of orphan drugs approved for market.
These incentives not only lower the financial burden associated with developing treatments for small patient populations but also mitigate the risks involved. As a result, more companies are entering the orphan drug space, leading to a surge in the availability of therapies for rare diseases. This dynamic is likely to enhance competition in the market, driving innovation and ultimately benefiting patients who rely on these specialized treatments.
Growing Investment and Partnerships in the Biotech Sector
The orphan drugs market is also experiencing a dynamic shift characterized by growing investment and partnerships within the biotech sector. As the potential for high returns on investment becomes evident, venture capital and private equity firms are increasingly directing funds toward biotech companies focused on orphan drug development. By 2025, it is expected that the influx of capital will facilitate the acceleration of R&D efforts, enabling companies to advance their pipelines more rapidly.
Additionally, strategic partnerships between established pharmaceutical companies and biotech firms are becoming more common, allowing for the sharing of resources, expertise, and technology. These collaborations are essential for navigating the complexities of orphan drug development, including regulatory approval processes and market access challenges.
Furthermore, as patient advocacy groups gain prominence, they are playing a vital role in raising awareness and driving funding for research into rare diseases. This collaborative ecosystem fosters innovation and enhances the likelihood of successful drug development, ultimately leading to an increase in the number of orphan drugs available to patients. As investment continues to grow, the orphan drugs market is poised for significant expansion, improving treatment options for those affected by rare diseases.
Orphan Drugs Market Report Segmentation:
Breakup by Drug Type:
· Biological
· Non-Biological
Biologicals account for 65.8% of the market share, making them the largest segment due to their effectiveness in addressing the underlying pathophysiological mechanisms of orphan diseases.
Breakup by Disease Type:
· Oncology
· Hematology
· Neurology
· Cardiovascular
· Others
Oncology accounts for 36.6% of the market share, maintaining a dominant position in the orphan drugs segment due to the high incidence of rare cancers and the growing demand for targeted therapies.
Breakup by Phase:
· Phase I
· Phase II
· Phase III
· Phase IV
Phase I of orphan drug development is primarily aimed at assessing safety, determining the appropriate dosage range, and analyzing pharmacokinetics in a small group of healthy individuals or patients with the targeted condition.
Breakup by Top Selling Drugs:
· Revlimid
· Rituxan
· Copaxone
· Opdivo
· Keytruda
· Imbruvica
· Avonex
· Sensipar
· Soliris
· Others
Keytruda (pembrolizumab) accounts for 15.7% of the market share and ranks among the top-selling medicines, particularly within the orphan drugs segment.
Breakup by Distribution Channel:
· Hospital Pharmacies
· Retail Pharmacies
· Online Stores
· Others
Hospital pharmacies account for 48.2% of the market share, making them the leading segment. Their dominance is largely attributed to their critical role in dispensing orphan drugs, which often require specialized handling and administration.
Breakup by Region:
· North America
· Asia Pacific
· Europe
· Latin America
· Middle East and Africa
In 2024, North America held the largest market share at 35.5%, driven by strong regulatory support, technological innovation, and an increasing emphasis on rare disease treatment.
Competitive Landscape with Key Players:
The competitive landscape of orphan drugs market size has been studied in the report with the detailed profiles of the key players operating in the market.
Some of These Key Players Include:
· AbbVie Inc.
· Alexion Pharmaceuticals Inc
· Amgen Inc.
· Biogen Inc.
· Bristol-Myers Squibb Company
· F. Hoffmann-La Roche AG (Roche Holding AG)
· Jazz Pharmaceuticals Plc
· Johnson & Johnson
· Merck & Co. Inc.
· Novartis AG
· Pfizer Inc.
· Sanofi S.A.
· Takeda Pharmaceutical Company Limited
· Teva Pharmaceutical Industries Ltd.
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=2382&flag=C
Key Highlights of the Report:
· Market Performance (2019-2024)
· Market Outlook (2025-2033)
· Market Trends
· Market Drivers and Success Factors
· Impact of COVID-19
· Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Website: imarcgroup.com
Email: sales@imarcgroup.com
Americas: +1-631-791-1145
- Questions and Answers
- Opinion
- Motivational and Inspiring Story
- Technology
- True & Inspiring Quotes
- Live and Let live
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film/Movie
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News
- Culture
- Military Equipments

