Enzyme Inhibitor Market Trends & Forecast 2025
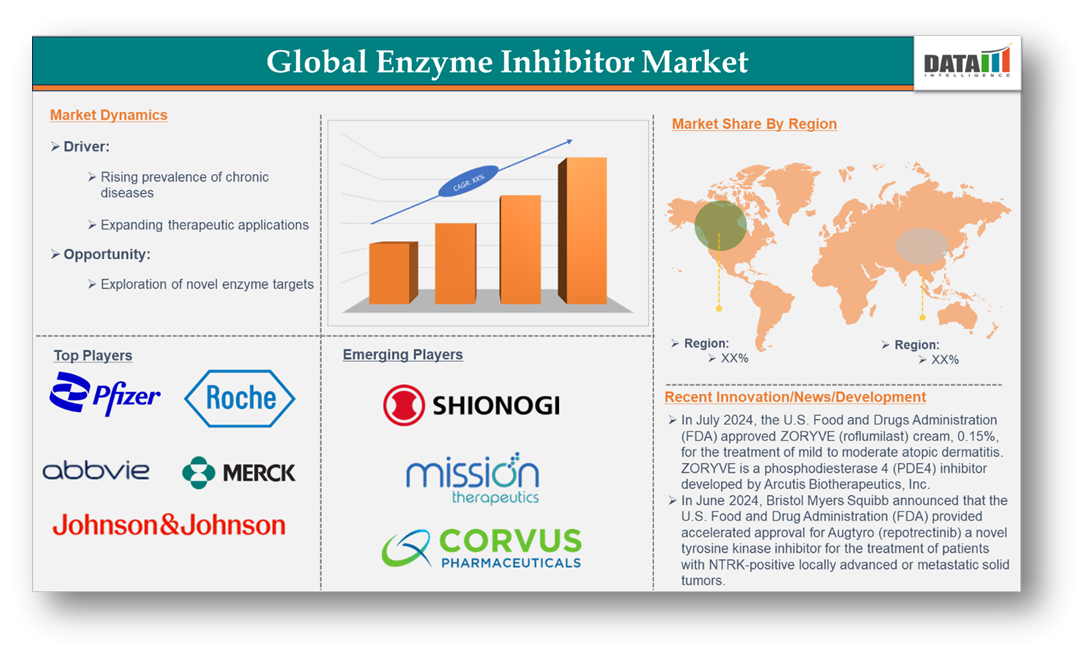
The Global Enzyme Inhibitor Market reached US$182.77 billion in 2023 and is expected to reach US$256.53 billion by 2032, growing at a CAGR of 3.9% during the forecast period 2024-2032.
The Enzyme Inhibitor market report provides in-depth insights and analysis on key market trends, growth opportunities, and emerging challenges. With a commitment to delivering actionable intelligence, DataM Intelligence empowers businesses to make informed decisions and stay ahead of the competition. Leveraging a combination of qualitative and quantitative research methods, the company offers comprehensive reports that help clients navigate complex market landscapes, drive strategic growth, and seize new opportunities in an ever-evolving global market.
Get a Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):- https://shorturl.at/RycS3
An Enzyme Inhibitor is a molecule that binds to an enzyme and reduces its activity. It can work by blocking the enzyme's active site or altering its structure. Enzyme inhibitors are used in medicine to treat diseases and in research to study biochemical processes. Examples include drugs like aspirin, which inhibits cyclooxygenase enzymes.
Forecast Growth Projected:
The Global Enzyme Inhibitor Market is anticipated to rise at a considerable rate during the forecast period, between 2024 and 2032. In 2023, the market is growing at a steady rate, and with the rising adoption of strategies by key players, the market is expected to rise over the projected horizon.
List of the Key Players in the Enzyme Inhibitor Market:
Pfizer Inc., Johnson & Johnson Services, Inc., Merck & Co., Inc., F. Hoffmann-La Roche Ltd, Novartis AG, AbbVie Inc., Sanofi, AstraZeneca, Gilead Sciences, Inc., and Eli Lilly and Company
Emerging Players
The emerging players in the Enzyme inhibitor market include Shionogi Inc., Mission Therapeutics, and Corvus Pharmaceuticals among others.
Industry Development:
In July 2024, the U.S. Food and Drug Administration (FDA) approved ZORYVE (roflumilast) cream, 0.15%, for treating mild to moderate atopic dermatitis in adults and children aged six and older. Developed by Arcutis Biotherapeutics, Inc., ZORYVE is a phosphodiesterase 4 (PDE4) inhibitor.
In June 2024, Bristol Myers Squibb announced that the FDA granted accelerated approval for Augtyro (repotrectinib), a novel tyrosine kinase inhibitor designed to treat patients with NTRK-positive locally advanced or metastatic solid tumors.
Contact Us -
Company Name: DataM Intelligence
Contact Person: Sai Kiran
Email: Sai.k@datamintelligence.com
Phone: +1 877 441 4866
Website: https://www.datamintelligence.com
- Questions and Answers
- Opinion
- Motivational and Inspiring Story
- Technology
- Live and Let live
- Focus
- Geopolitics
- Military-Arms/Equipment
- Sicherheit
- Economy
- Beasts of Nations
- Machine Tools-The “Mother Industry”
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film/Movie
- Fitness
- Food
- Spiele
- Gardening
- Health
- Startseite
- Literature
- Music
- Networking
- Andere
- Party
- Religion
- Shopping
- Sports
- Theater
- Health and Wellness
- News
- Culture

